The Israeli Ministry of Health Actually Did a Survey of Adverse Events after The Booster Dose
And it's absolutely devastating
Well surprise, surprise. The Israeli Ministry of Health (MoH) conducted an actual survey of about two thousand people 3-4 weeks after they received the third (booster) shot of the Pfizer/BioNTech COVID-19 vaccine, asking them about the adverse events they experienced following vaccination. The results are absolutely devastating to the COVID-19 vaccine program and the push for vaccine mandates. They come to me from my brother-in-arms. ‘Galileo is Back’ (and boy is he pissed!).
The survey report was released last week (Feb. 9) and from what I could find it is not available on the MoH website but only via a website for medical staff only, so it is not publicly available. (Correction: it was released on the MoH Telegram channel.) The report was greeted by the media mainly with near dead silence, aside from a couple of articles downplaying the adverse events as mild and transient.
This is after more than a year that every talking head on TV and every newspaper large and small assured the Israeli public that they were monitoring the safety of the vaccines and that they were obviously extremely safe because there were very few reports and most of them were extremely minor and you were a crazy conspiracy theorist to doubt it.
Of course the MoH, along with the CDC, completely ignores the unambiguous and unprecedented safety signals emanating from the US Vaccine Adverse Events Reporting System (VAERS). Never mind that the passive, voluntary adverse events reporting system that the MoH put into place, which basically nobody knew about, was a complete farce. They took a page from the Pharma playbook by deliberately failing to gather data appropriately so they could turn absence of evidence into evidence of absence.
The MoH didn’t have to look further than their own Facebook page to find plenty of evidence. On Sept. 30 they put up a Facebook post deriding all the “fake news” on social networks about side effects and touting the safety of the vaccines: only 19 serious adverse events reported out of 3 million booster doses, which might not even be related to the vaccine. The post was quickly flooded with thousands of comments from people describing in detail the severe adverse events they or their loved ones had experienced after the booster and previous doses (see here for automated translation of many of them). Somebody at the MoH apparently panicked, because they quickly started deleting hundreds if not thousands of comments. They were caught ‘in the act’ by people reading the comments who took videos of them being deleted, one by one. There are currently 27,000 comments on that post. It is unknown how many were deleted.
And apparently it had never occurred to the MoH to investigate why so many people never returned for the second dose, despite losing eligibility for the green pass or the right to work in countless workplaces requiring vaccination. One careful calculation I’ve seen estimated that by June 2021, there were about 180,000 people who had never returned for the second dose—a number that excludes people who recovered from a SARS-Cov-2 infection. That’s over 3% of people who were vaccinated with one dose and eligible for the second at that time.
At the FDA advisory committee hearing on Sept. 17 to decide whether to approve the booster shot, Israel’s data on the 3rd dose played a starring role — including their safety data. It was extremely safe, they assured the FDA, as they had received so few reports, even less than the first two doses! And even most of the ones they did receive were not caused by the vaccine, including this woman in her 60’s who had a stroke immediately after receiving the booster dose. Why wasn’t it causally related? Because they said so.
While the FDA panel applauded Israel for being an exemplary laboratory (I wish I was joking), they nevertheless decided that the Israeli data was insufficient to approve the booster for people under 65. The MoH was undeterred. Thanks in part to the new policy requiring a third dose for “green pass” eligibility that prompted many reluctant young Israelis to go in for dose #3 along with the army forcing it on draftees, they were able to return to the FDA the following month (Oct 14) with more data they had gathered on the effectiveness and safety of the booster dose in younger populations, again with the same conclusion: extremely safe. Like so, so safe. Never mind all those pesky Facebook posts and people who never returned for dose 2.
Meanwhile, the survey they were conducting on adverse events after the booster, which paints a completely different picture about its safety profile, was wrapping up and would be finished less than two weeks later on Oct. 25th. It’s worth noting that the MoH had ample time to analyze the survey data before recommending the 4th booster dose on January 25.
So what do the survey results tell us about the safety of the booster dose? And what can they tell us about the extent to which the existing Israeli vaccine safety monitoring system was undercounting adverse events? And can we use them to estimate the underreporting factor for some events in VAERS? (Short answers: horrible safety, massively undercounted, and yes we can!)
Here are some of the most important takeaways from the survey. Further down I’ll present all the results and conclude with some discussion of unreported events:
0.5% of people who reported an adverse event reported being hospitalized as a result of the adverse event they experienced (that’s 0.29% of everyone in the survey). Not just the emergency room, but actually being hospitalized. Think this is impossibly high? Read this footnote.1 If the numbers are wrong, the MoH needs to say so and explain how they messed up. If we use the 0.29% rate for the entire sample, we can extrapolate from the survey to estimate the number of hospitalizations associated with adverse events within a month of receiving a booster dose:
Israel has administered about 4.5 million booster doses so far, which equates to over 13,000 hospitalizations.
Over 92 million booster doses have been distributed in the U.S., which equates to nearly 270,000 hospitalizations.
Read this footnote for some methodological caveats.2 [I have gone back-and-forth in different versions of this article between reporting the hospitalization rate as 0.5% or 0.29%. The report clearly states that this is the rate among everyone reporting an adverse event (6 out of 1,140). It does not state whether there were additional hospitalizations among respondents that were not associated with the adverse event. A back-of-the-envelope calculation indicates that a 0.29% hospitalization rate is in line with what we would expect to find among a random sample of Israelis. So if the 0.5% rate among people with adverse event includes all the hospitalizations in the entire survey, then it is more or less in line with expectations. But if it is in addition to other hospitalizations, then it points to a significantly higher hospitalization rate following the booster.]
29% reported that they had difficulty performing daily activities as a result of the adverse event. That is 44% of the 66.4% of the sample that reported at least one adverse event. Like in VAERS, women were more likely to report than men (75% vs. 58%) and also more likely to report difficulty in daily functioning (51% vs 35%).
4.5% of respondents reported neurological problems (again, more women than men), including:
Bell’s Palsy (0.5%)
Blurred or disturbed vision (0.5%)
Seizures/Convulsions (0.15%)
Loss of consciousness (0.2%)
16% of these neurological problems occurred within an hour of vaccination, an additional 27% within 24 hours, and 47% were ongoing by the time of the survey 3-4 weeks after vaccination.
In the US, this translates to 460,000 cases of Bell’s Palsy and blurred or disturbed vision, 135,000 seizures and 180,000 people losing consciousness.
Have you ever heard of a vaccine that is associated with seizures in 0.15% of adults who take it — let alone one that is mandated by many colleges and workplaces?
About 25% of people with pre-existing auto-immune disorders, depression or anxiety reported a worsening of their symptoms following the booster. Five to ten percent of people with diabetes, hypertension, and lung & heart disease also reported a worsening of their condition.
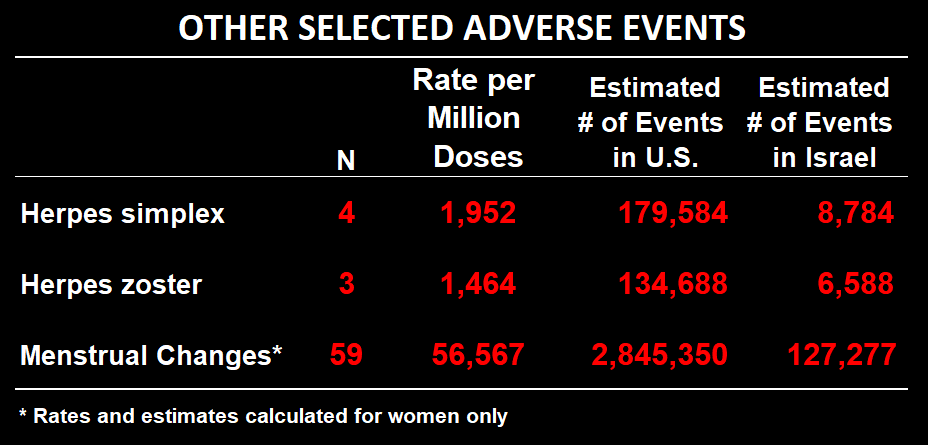
Nearly 10% of women under age of 54 had disruptions to their menstrual cycle after the booster (apparently they did not ask women above this age). About half of those women reported that the problems persisted at the time of a follow-up interview, which was anywhere from 10-16 weeks after vaccination.
Of these, 31% sought medical treatment as a result and 9% were on medication for it.
Notably, 39% of these women reported similar disruptions following prior doses, of whom 1/3 (which is a little over 6% of all women under the age of 54) were still experiencing symptoms at the time of the 3rd dose. Given that the vast majority of vaccinated Israelis were “fully vaccinated” by the end of March and the booster campaign for that age group didn’t get into full swing until late August, this means that these women were likely experiencing these symptoms for somewhere between 4-months.
How badly did the Israeli vaccine adverse event reporting system undercount adverse events? We can calculate an approximation by comparing the MoH’s Sept. 25 report on adverse events from this system to the survey results to calculate an “underreporting factor” (URF). If the URF is 100, this means you have to multiply the number of reported events by 100 to approximate the true number of adverse events.3 It’s especially important to know the URF when public health officials disingenuously play down the risks of a medical product by saying that there have been very few reports of adverse events, while knowing full well that the true number must be much larger. The only question is — how much larger?
The URF varies from a low of 1,700 for loss of consciousness to 48,800 for difficulty breathing. Some other highlights: a URF of 6,500 for seizures, nearly 6,000 for Bell’s Palsy, and over 4,000 for blurry or disturbed vision.
Actually the URF was even higher for some milder, general AE’s and for local site reactions: 54,000 for chest pains, 230,000 for limited arm movement, and 540,000 for injection site pain. That these types of AE’s are so underreported is hardly surprising: the public was told to expect these kinds of reactions, and for the most part they are relatively minor (though note in the last table below how long some of them persist for).
Note that these URF’s cannot be applied to VAERS, for two reasons:
The underreporting in Israel is probably much worse than in the US for a variety of reasons.
Israel does not conform to the international classification standard for AE reporting. The US, UK and Europe use the MedDRA system. The Israeli MoH apparently decided to make up its own classification system and continues to use it, for reasons unknown. So comparison to other countries is difficult for some of the events, but easier for others.
I’m going to use the survey results to estimate VAERS underreporting for four relatively serious and specific adverse reactions: seizures, hospitalization, Bell’s Palsy, and shingles (herpes zoster).
VAERS URF for Seizures/Convulsions is 731:
As of Feb. 11, there were 243 reports of seizures and convulsions reported in VAERS that occurred within 30 days of receiving the booster dose in the US and territories (143 of these were after Pfizer).
As of Feb. 11, about 91 million booster doses had been administered in the US, of which about 50 million were from Pfizer.
The rate of per million doses of seizures/convulsions in the MoH survey was 1952 (see footnote 3 for method of calculation).4
From that rate the expected number of seizures/convulsions after the booster in the US by Feb. 11 is 177,632 (=1952*91).
177,600/91 = 731. If we want to make a strict apples-to-apples comparison, we need to look at reports after Pfizer only, in which case the URF is 683.
VAERS URF for Hospitalizations is 126:
As of Feb. 11, there were 2,120 hospitalizations in VAERS (1,207 for Pfizer).
The rate of hospitalization in the MoH survey per million doses was 2,928.
The expected number of hospitalizations after the booster is 266,488.
266,488/2,120 = 126. For Pfizer it’s 121.
VAERS URF for Bell’s Palsy is 3,034:
As of Feb. 11, there were 161 cases of Bell’s Palsy listed in VAERS (95 of these were after Pfizer).
The rate of Bell’s Palsy in the MoH survey per million doses was 5,368.
The expected number of cases of Bell’s Palsy after the booster is 488,488.
488,500/161 = 3,034. For Pfizer it’s 2,825.
VAERS URF for shingles (herpes zoster) is 401.
As of Feb. 11, there were 332 cases of shingles reported in VAERS (196 after Pfizer).
The rate of shingles (herpes zoster) in the MoH survey per million doses was 1,464.
The expected number of shingles cases after the booster in the US is 133,200.
133,200/332 is 401. For Pfizer only it’s 373.
Here is a table summarizing the URF calculations for these four AE’s:
Do you know of any other mandated medical treatment or vaccine that is associated with so many problems in such a high proportion of the population? For that matter, how many mandated medical treatments have you ever even heard of? The results of this survey should have put an immediate end to the plans of governments around the world to continue to offer booster doses to their citizens, let alone condition basic liberties on getting one. And yet, here we are. The only question now is how much lower this report can limbo under the radar.
Below are tables showing the number and percent of reports from the survey and comparing those results, where possible, to the MoH report based on the passive surveillance system. I’ve also calculated the estimated total number of events that would be expected to occur in the US and Israel based on the survey reporting rate per million and the total number of booster doses administered in each country, based on approximately 92 million doses administered in the US and 4.5 million in Israel. Of course these are rough estimates, and the survey itself has a margin of error of +/2%. The full tables, including a gender breakdown, are available here. To read more about how the URF was calculated, read this footnote.5 I’m not going to add much commentary on the tables as they sort of speak for themselves. I’m also not going to show a table for local administration site reactions, as they are relatively less sever and less interesting (though note in the last table how long many of them last for).
Let’s start with some of the more serious AE’s, starting with Neurological:
Here is the table for allergic reactions. They say no cases of anaphylaxis were reported during the survey, but difficulty breathing and throat swelling are anaphylactic reactions, even if they do not reach the severity of full-blown anaphylaxis.
Here are reactions classified as “general:"
Here is a table showing what percentage of respondents had pre-existing chronic illnesses and, of those, what percentage experienced a worsening of symptoms after the booster dose:
Wait, weren’t the vaccines were supposed to protect people at greater risk of severe COVID outcomes, not make them worse?
Here are three AE’s that were not reported by the MoH in its report on its spontaneous AE monitoring system, so a URF cannot be calculated (Note: table is corrected from earlier draft):
A follow-up study of the women who reported menstrual changes was conducted 7-12 weeks after the first interview. About half of them were still experiencing problems at the time of the follow-up. Here is the breakdown of the kinds of changes they experienced (categories are not mutually exclusive).
Finally, here is a table reporting how soon after vaccination they experienced the adverse event for different categories and the duration of the symptoms. Note that a sizeable percentage of symptoms are listed as “ongoing,” meaning they were still experiencing symptoms from the adverse event at the time of the interview 3-4 weeks following receipt of the booster dose.
A sizeable percentage of events began on the same day as the vaccination. But as we saw at the top, even if you have a stroke the moment the shot goes into you, it may still be ruled a “coincidence” by the wise sages in the Ministry of Health. To them I say: don’t piss on my leg and tell me it’s raining. They’re engaged in an exercise of ghoulish gaslighting, and I honestly wonder how they sleep at night. To give them the benefit of the doubt, instead of being motivated by malevolence their actions might be due to arrogance allied with ignorance: since they don’t know how the vaccines could possibly cause a particular problem, then obviously they couldn’t have. Because they know everything. Apparently. And you dare not question them or you will be shunned from polite society.
I will save a deep-in-the-weeds methodological discussion for the final footnote,6 but it’s worth pointing out as I wrap this up that the survey is also notable for the events it doesn’t include. They did report one case of myocarditis. According to Israeli figures, the highest rate of myocarditis following the second dose for every 6,600 boys aged 16-19 and significantly less for older people and females. So it is quite remarkable that even 1 case was observed in this survey of 2,049 people, few of whom were in the highest risk category for myocarditis. This means either that it was a fluke or that the chances of myocarditis are significantly larger following the booster dose.
It also means that to detect the events they did for relatively rare conditions like Bell’s Palsy or seizures means that these events must be far more common than myocarditis, at least among the population under study in this survey. (And VAERS has over 11,000 unique types of events that have been reported for COVID-19 vaccines.) At a later date I may do a power analysis on this survey to try to estimate the likelihood of detecting certain types of events, but even without that it is stating the obvious to say that the survey was unlikely to pick up any event that was less likely to occur than, say myocarditis. But since we’re talking about millions or even billions of vaccinations, even events as rare as 1 in 10 or 20 thousand equate to hundreds or hundreds of thousands of cases.
One of the other things you won’t see in this survey is the percentage of people who died within a month of being vaccinated, for the simple reason that you can’t phone up dead people to find out what happened to them after vaccination, unless perhaps you use a Ouija board. But I haven’t seen a legitimate survey that uses that kind of methodology. On the other hand I haven’t seen a legitimate adverse event report organized using the MoH’s categories, so maybe they’d be up for it. Frankly, I’m surprised they did this survey in the first place, and even more surprised that they released this report at all. It will be interesting to see how they will tie themselves up in knots to deny the results of their own study. Or maybe they’ll just ignore it and hope it goes away. It’s up to us to make sure that doesn’t happen.
As a send off, here are some bar charts summarizing some key takeaway points:
Some people have said the rates reported here for some events are impossibly high. And I agree they seem almost unbelievably high. But they are in line with figures from the CDC’s v-safe program, which is the closest thing they have to a survey. It’s an app that people can voluntarily download to their phone after vaccination, and it nudges them to answer questions at certain intervals after vaccination. (Apparently, Israel “start-up nation” was unable to do even this.) Based on v-safe data, the CDC reported an overall hospitalization rate within a week of the first dose of 0.1%, which was also the rate for people who received a booster and primary series all from Pfizer only. Overall, 28% were unable to perform normal daily activities, or 22% of the ‘Pfizer only’ group (this compares to 29% in the MoH survey). 1.2% of v-safe respondents developed a non-injection site rash (1.9% of ‘Pfizer only’) vs. 2% in the MoH survey. Other results from v-safe are also quite similar to the MoH survey, and overall the similarities lend credibility to the validity of the survey results.
However, it should be noted that that MMWR report was based on boosters received by about 22,000 immuno-compromised individuals. A recent MMWR report (dated Feb. 18) on non-immunocompromised individuals enrolled in v-safe who received a booster (N is about 720,000; or 332,000 ‘Pfizer only’ — however less than 1% of booster recipients registered in v-safe). But the report is very light on details about specific adverse events. We can compare ‘systemic’ reactions to the MoH’s ‘general reactions’ where we see the CDC reporting 58.4% among ‘Pfizer only’ vs. 48.6% in the MoH survey. These numbers are 64.3% vs. 55.7% for local injection-site reactions. Beyond that, we can glean that 0.9% of Pfizer-only recipients sought medical care following the booster dose, but we don’t know what the rate of hospitalization was. Nevertheless, the other comparisons suggest the estimates from the MoH survey are lower than from the CDC v-safe data. Note that the CDC has resisted FOIA requests for more detailed breakdowns of the v-safe data, though it is known that they possess the requested information.
A clinical trial testing ‘mixing-and-matching’ boosters published in the NEJM can also be used as a point of reference, although there were only 50 people in the “Pfizer only” group. Among that group, the trial reports higher rates of myalgias, arthralgias, headaches, nausea and injection site pain than the MoH survey, again suggesting the MoH survey may be underestimating the rates of adverse events. Unfortunately there is no further detail about adverse events reported, and in any case they only have an N of 50 in the Pfizer-only group.
For menstrual disruptions, we can compare to a survey that was conducted in the UK in March of 2021. They found that 20% of women who responded to the survey had experienced menstrual changes following vaccination. That was after the first or second dose, not the booster. The difference between that and the 10% found in this survey could be due to multiple factors: 1. In the UK many women received the AstraZeneca vaccine; 2. Respondents were recruited through a Facebook campaign, and women whose menstruation was affected adversely by the vaccine might have been more motivated to respond (even though the survey was not specifically about that); 3. Women who experienced menstrual disruption after the 1st and 2nd doses may have been less likely to go back for the 3rd, which would skew the distribution of women receiving the 3rd shot towards women less likely to be affected.
Obviously there are inherent limitations in trying to extrapolate the numbers from this survey to the broader Israeli and American populations. First of all, every survey has some degree of uncertainty, which can be calculated with the raw data. But we don’t have the raw data, and the MoH did not supply that information, so we are in the dark. Clearly these numbers are not exact, but even if they are in the same ballpark they are huge. And there is no reason to think otherwise.
Some people have said the raw numbers are too small (e.g., 4 seizures) to make anything of. This isn’t true. Call up 2,049 adults at random and ask how many had experienced seizures in the previous 4 months. I think most of the time you wouldn’t get any, let alone 4. But it has been said that these data do not have background rates so it can be difficult to judge. I’m working on that.
Another point here is that if the MoH wants to study adverse events but believes they are really rare, then why did they field such a small survey? What kind In June they ran a survey on kids who had ever tested positive for SARS-Cov-2 that included over 13,000 respondents. Here, they started with a sample of 4,321 people who met their inclusion criteria (which was too small in an event), but then did not even attempt to contact 1,427 of them, for reasons unknown. So we are left with a small sample with relatively few events for more serious conditions, which people then brush aside with the wave of their hand. It’s almost as if the survey was set up to fail.
Finally, there is an issue with the survey design. The survey was stratified by age, which means it was not perfectly representative, age-wise, of the people who actually got the booster (either in Israel or the US). We don't know how non-representative it was because they don't report on this. I couldn't even see how to reverse engineer the age-breakdown of the survey. Normally one corrects for stratification by weighting and other things when reporting survey results. As far as I can tell the MoH just reports the raw numbers, not weighted. So it's not straightforward to just multiply the rate by the number of boosters to figure out the expected number of events, because it can be expected that the population getting boosters skews older than the sample, so in theory there would be a lower rate among total boosted. I don't know how much lower. I don't expect it's a ton lower, or even an order of magnitude, but we can't know without more information on the survey.
So yes, we have to take these projections with several grains of salt. I calculated them to illustrate the magnitude of the results. Even if they are significantly off, they are still big enough to raise huge alarm bells.
Yes, it’s true that adverse events reported either through the safety monitoring system or in the survey are not necessarily caused by the vaccine. That doesn’t matter, because monitoring systems are not meant to determine causality (though they arguably can). Their purpose is to collect reports on adverse events experienced after a medical intervention, regardless of whether the person reporting thinks there is a causal relationship. If people don’t report, then they don’t work as intended. Simple as that.
There should actually be more cases, because the survey included people who had received their booster 3-4 weeks earlier. Here I am including both reports and vaccination numbers through Feb. 11. That means that for somebody vaccinated on Feb. 11, they still have 4 weeks to have experience an adverse event and report to VAERS in order to be included in the count of cases. This is therefore a conservative estimate. I could have constrained VAERS to an earlier vaccination date (say, Jan 11), but there are so many missing fields in VAERS data that doing so would likely exclude many cases with an unknown vaccination date.
The survey results were based on responses from 2,049 Israelis. The MoH reports based on passive surveillance were reported as events per million doses. You have to multiply 2,049 by 488 to get to a million. So the number of each type of event was multiplied by 488 to approximate the total number of reports per million that should have been documented in the passive system if there was no underreporting (on the assumption that the survey is not underreported). Another option would have been to multiply the percentage by 10,000 to get the rate per million. The problem is that for many events the number of events did not line up with the percentage. What I mean by that is that if you took the number of events reported and divided by 2,049, the result did not quite equal the percentage reported by the MoH. I can only guess as to why this was. Perhaps for each question they excluded people who didn’t answer the question and only used those who provided an answer as the denominator. I don’t know and it isn’t written. However, since we know the full sample size, using the total number of people who reported an event as our baseline is a sound methodological choice. In any case the differences between the two methods yield very similar numbers and reveal the same magnitude of underreporting. I thank Oz Koren for laboring to compare the reports and calculate the URFs.
I’ve been plugging away at this post for most of the day, and I’m running out of steam. I will make two quick methodological notes here and perhaps add some more at a later date. First of all, how precise are the results from the survey? It isn’t reported. Usually with a survey they report a margin of error, typically +/- 3%. That means that if the percent of people saying X is, say 10%, you can be confident that if you did the same survey on a different random sample, you would get a number between 7 and 13 at least 95% of the time. Here there is no margin of error, so we are left to wonder. The survey was carried out by a reputable survey research firm, so we may assume that the margin of error is within that range, if not smaller. I could probably do the calculations myself if I had the time.
Another thing I want to touch on is non-response bias. First of all, they sampled from the complete list of people who were registered as having taken the booster, of whom only 75% had telephones. I don’t know any child in Israel above the age of 5 without their own phone, so I have to wonder who these 25% are without phone numbers listed. But set that aside. Of the people with phone numbers, there were a sizeable number of people they were either unable to make contact with (347) or who refused to be part of the survey (469). If those people were more likely to have suffered or be currently suffering from some serious adverse event, then the survey would tend to undercount those events. For example, if you are dealing with a severe health problem, you might be less able to answer the phone or less willing to talk to representatives of the Ministry of Health if you are angry at them. This is pure speculation, of course. It’s impossible to know whether and how the non-response bias could affect the results, if at all. If it’s random, then it won’t. If it skews towards people who had an AE, then it will lead to an undercount. If it skews towards people who didn’t have an AE (nothing happened to me, why waste my time talking to them?), then it will lead to an overcount.
We can calculate a lower bound for our estimates if we assume that everybody who was contacted but did not participate (either by refusal or not picking up the phone or not completing the survey) experienced no adverse events. In that case the survey size would be 2,894 but the counts of events would remain the same. So the hospitalization rate would have been 0.2%. the shingles rate 0.1%, the seizure rate 0.14% and the Bell’s Palsy rate 0.38%. These are still huge numbers: 2000, 1000, 1400 and 3800 per million doses.















If these transfections can be considered 'safe and effective' then, by that same standard, getting covid naturally would be 'safe and extremely effective'
Wow. This is the first of your articles I have read, and I have just subscribed. I very much appreciate your well researched, unhyped, unbiased data and analysis. Well done! Thank you.