Is Subject #12312982 the Key to Proving Pfizer Vaccine Trial Fraud?
The Story of Augusto Roux
Subject # 12312982 in Pfizer study C4591001 is Augusto Roux, a 35-year old lawyer from Buenos Aires, Argentina who volunteered for Pfizer’s stage 3 trial of its COVID-19 vaccine (or whatever you want to call it) in order to protect his mother with emphysema.
His story and some of the shenanigans surrounding the Argentinian trial site have been amply covered by Dr. David Healy in three sprawling but extremely important blog posts: 1, 2, 3. The first one was published March 1st, but it was only last week that I caught on to this story, so I’m assuming most of you probably aren’t familiar with it. So please share this — we’ve got to get the word out, because Augusto Roux may very well hold the key to bringing down the Pfizer vaccine trial, or a least proving fraud at the largest trial site that was home to over 10% of the participants in the trial.
[UPDATE FEB 23,23: David Healy recently published a paper in a peer-reviewed journal detailing COVID vax trial volunteers Augusto Roux and Bri Dressen’s stories, “The coverage of medical injuries in company trial informed consent forms.” — Huzzah for David Healy!]
If you don’t have time to sink your teeth into David’s posts (even just the 1st one), I’ll bring you up to speed on key details:
On the way home after his second dose on Sept. 9, 2020, he began feeling unwell, developed a high fever and felt terribly ill until he fainted on Sept. 11 and finally went to the hospital on Sept. 12 (not the one where the trial was being run). They did a thorough work-up, including a CAT scan of his chest that showed an abnormal collection of fluid around the outside of the heart. Basically he had pericarditis.
On Sept. 14, he was discharged. The doctor wrote in his chart that he had suffered an adverse reaction to the vaccine. Augusto was told by hospital staff they there had been a huge influx of people from the clinical trial coming to the hospital (there were 2,981 subjects enrolled in the trial before Augusto), so his experience was not new to them. (The trial site managed to enlist several thousand subjects in just a few weeks.) One nurse estimated they had seen around 300 people.
Now here’s where it gets really interesting, and we know all of this because Augusto, a lawyer, successfully sued to get his medical and trial clinical records, even though it took him over a year. I will try to make a long story short:
Even though Augusto had a negative PCR test at the hospital, and even though the doctor at the hospital wrote that his condition was due to the vaccine, when Augusto called the trial site on Sept. 14 to notify them he was in the hospital, they wrote down in his clinical trial record that he had been admitted for a bilateral pneumonia that had nothing to do with the “investigational product” — even though that was not what he told them.
On October 7, the clinical trial notes that “at the request of the sponsor” (AKA BioNTech), the adverse event code was update to suspected COVID-19 disease. And that’s how Pfizer/BioNTech made cases of myocarditis and pericarditis disappear, by sweeping them under the rug of suspected COVID-19. Moreover, the diagnosis of suspected COVID-19 would not count against the efficacy calculations, since those required a positive PCR test to confirm diagnosis.
Two days later On Oct. 9, Augusto was formally unblinded. The principal investigator for the trial, Fernando Polack (pictured below), had told him that Augusto could only be unblinded if his life were in danger, which is simply untrue. So Augusto appealed to ANMAT, the Argentinian FDA. In a formal hearing they forced the trial investigators to tell Augusto if he had received the vaccine or not. He had. If you think the timing of the Oct. 7 request by Pfizer to change his AE is suspect, you’re not alone.
A day before the hearing (and a day after the change in AE status), Polack wrote in Augusto’s clinical trial records that he had had an attack of severe anxiety starting on September 23, not caused by the vaccine, and wrote that Augusto suspected a conspiracy between the two hospitals, described his anxiety as constitutional, and noted that it was ongoing.
To add insult to injury, two days after the ANMAT hearing, Polack had the mental health diagnosis added to Augusto’s actual medical records. Of course a pediatrician like Polack has no business making mental health diagnoses, especially without any formal assessment.
Recall that Polack was the first author on the December, 2020 NEJM paper on the safety and efficacy of the vaccine. He is also one of the directors of i-trials, the site management organization paid handsomely by Pfizer to run the trial in Argentina (the largest site of the trial by far). If he raised an alarm about the vaccine safety, his company would have lost a ton of money and would be an unlikely choice by any company to run any trials in the future. So to say that he had an interest in achieving a positive trial outcome would be quite an understatement. There may be other conflicts we’re not aware of.
I spoke with Augusto at length over zoom last week. Such a nice man. [EDIT MAY 25, I deleted something I had written here at Augusto’s request. It’s not because it’s wrong, but rather because he wants to nail down the proof of the claim before going public with it. Suffice it to say that he has other information about severe adverse events that were covered up by the trial investigators. But his well-document case alone is enough to sink the ship.]
Augusto wrote to the FDA and its European counterpart, the EMA, in late 2020 explaining everything. He was ignored. He also filed a VAERS report in November, 2020, and received a confirmation with a temporary VAERS number. However his report never made it into the system as far as we can tell.
Now, the last set of files released as part of the FDA FOIA document dump included several data files from the trial, including a dataset with all of the protocol deviations recorded during the trial. These occur whenever the study protocol is not followed, and they are supposed to be recorded.
So what do these records indicate for Augusto? Here are the 3 deviations listed:
2020-09-12 Nasal swab not collected for the visit where it is required
2020-09-12 Visit performed outside of protocol specified window.
2020-10-09 Blind compromised
We know that Augusto did not visit the trial site on Sept. 12 because he was in the hospital. Apparently the “visit performed” they refer to is his hospitalization. And while it is technically true that the trial site did not collect the swab for the PCR, a swab was collected and a test done, just not at the trial site. And it correctly records his blind as having been compromised on Oct. 9. But, oddly, in the field reserved to record the ‘unblinding date,’ none is given. Furthermore, even though the unblinding should have been grounds to exclude Augusto from the trial efficacy analyses, it was not marked as important or as something worthy of exclusion.
There are many fishy things in the protocol deviation data, which I will be writing about soon. So, as they say, watch this space!





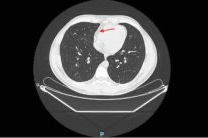


"nothing to do with the “investigational product”"
Very common dismissal.
Billions and decades of research later, doctors don't know the cause of autism, allergies, asthma, autoimmunity but they magically know trial adverse events are not related to this new mRNA vaccine?
https://vinuarumugham.substack.com/p/billions-and-decades-of-research?s=w
That Argentinian trial site keeps coming up.The first mystery was how they managed to get so many participants enrolled in such a short time. And now this. And you make an important point about these sub contractor sites wanting a positive outcome in order to get more work in the future. Surely Ventavia wanted the same. It would be so interesting to know the real AE numbers.